
Vitamins
Research has shown that in the late nineteenth and early twentieth centuries, the food factor must be involved in diseases such as beriberi, scurvy, and rickets. Dr. Casimir Funk, while reviewing the results of research in this field, in an article published in 1912, specifically stated that these diseases are caused by a lack of certain nutrients in the diet of patients with these diseases. Since Dr. Qutt himself was working at the time to extract a substance from yeast, a coating on rice grains, and other foods to treat beriberi, and a substance that had tissue in it in relation to nitrogen, he named the vitamin in that article. (Vitamine) suggested important ingredients for this group. Here, the first part of the name was taken from the word Vi - meaning vital, and the second part, the word arnine, meant to have nitrogen; So the word vitamin had a vital meaning. Although the word "vitamin" has been accepted and used in the scientific community since then, it has not been widely accepted in the United States. Subsequent research has shown that these nutrients are all nitrogen-rich: Professor Drew from the University of London in a short article. In 920, it was rumored that if the letter was removed from the end of the word vitamin, the amino and nitrogen meanings of the drug would disappear, so the word vitamin could be used as a vitamin. The name vitamin has been coined in the same way in food and nutrition culture and has been established. Vitamins are fundamentally different in terms of solubility. This means that some are soluble in fat and some in water. This difference is the basis for the division of vitamins into two groups: fat-soluble vitamins and water-soluble vitamins. Among these, vitamins E, B, A and K are fat-soluble and the rest are water-soluble. Excessive intake of water-soluble vitamins cannot be a problem for humans because they are excreted in the urine due to their solubility in water, but consuming large amounts of fat-soluble vitamins can cause problems if they remain in the body. Be. Problems with vitamins E and K are less likely, but vitamin D can be more problematic than other fat-soluble vitamins. Table 1 shows the required amounts of different vitamins in human daily nutrition
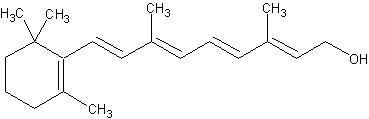
Vitamin A.
This vitamin is structurally a diphtheria pen, meaning it is composed of four isoprene units. In nature, there are alcohols or mainly esters of fatty acids. Its main source is beta-carotene It depends on the effect of enzymes, water activity and storage temperature of food or storage. It should be noted that pasteurization does not destroy vitamin A, but the presence of light has devastating effects on it. The amount of vitamin E in milk in summer is more than 1.5 times that in winter. The reason for the multi-difference is due to the nutritional status of livestock in these two seasons. Because in the summer, the beta trap receives more work and body through the material it feeds. That's why spheres produced in summer have a clearer yellow color than those produced in winter. And the number of these two substances of vitamin M is good. It is more than these vitamins and vitamins
Vitamin D.
This vitamin can be present in various forms, the most important of which are vitamin D or its ergocalciferol vitamin D3 or total calciferol. Vitamin D, like vitamin A, is not found in plant products. Vitamin D3 is widely found in animal products, but in large quantities only in fish liver oils. Vitamin D2 levels in fish oils are low. The precursors of vitamins D2 and D are ergosterol 13 and 7 - dehydrocholesterol 14, respectively. If these substances are exposed to ultraviolet light, they become the mentioned vitamins. Such conversion can also be caused by sunlight. But it should be noted that the specific wavelength of sunlight that is able to do this, and the best wavelength for it is 280 nm - can not pass through the glass, and in this respect the sun's radiation from behind the glass is fruitless. Figure 3 shows the structure of vitamins D and D2 and their precursors. Vitamin D performs three physiological functions in the body, which are (1) the absorption of calcium and phosphorus from the intestine, (2) the movement of calcium and phosphorus from the bones, and (3) the absorption of vitamin D with a decrease in that fruit. In addition to its specific vitamin effects, the ability to lighten tocopherols has specific antioxidant properties, and in fact, attention to tocopherols in foods is more related to their antioxidant properties. That is why it is always recommended to inflict the least damage to the tocopherol in the industrial oil production process; It plays an important role in the stability of the oil against oxidation in later stages and during oil storage. In this connection, the OH attached to the ring performs the antioxidant action. If the oil treatment is done well, there will be no reduction in the amount of tocopherol. Tocopherols are generally unstable to ultraviolet radiation but are relatively stable to heat, acid, and alkalis, especially in the absence of oxygen. In the past, it was believed that if the amount of tocopherol in the oil (when added as an antioxidant) exceeds a certain level as a peroxidant or case, physical action is an important and dominant process because the rate is 100% of the chemical reaction. . In the physical state, the tocopherol removes oxygen from the form of the stimulated unit and converts it into ordinary oxygen, which has the ability or low power of a destructive reaction ram. It is important to note that in this case, the ability of tocopherols to counteract oxidation is reversed when they act as normal oxidation associated with the formation of free radicals; That is, it is related to their vitamin properties. Although the consumption of oils containing polyunsaturated acids, especially omega-3 acids (such as fish oil), is beneficial in terms of maintaining health and is highly recommended, research in recent years has shown that excessive consumption of such oils reduces the amount. It contains vitamin E in the body, which should be due to the antioxidant role of this vitamin. In this regard, experts in the use of these oils have even suggested that in order to prevent the adverse effects of vitamin E deficiency, along with the use of these oils, | Vitamin E capsules should also be taken. Studies in recent decades have shown that one of the major causes of age-related diseases is the accumulation of free radicals due to the oxidation of fats in the body. Therefore, tocopherols can play a valuable role in this regard. In biological systems, the combination of ascorbic acid with tocopherol increases the antioxidant function of tocopherol. Of course, the cause of this condition is not the action of ascorbic acid as an independent antioxidant? Instead, as a mediator, ascorbic acid takes hydrogen from NADH, giving it the vitamin A that hydrogen gives to a free radical formed by oxidation and inactivating itself. In this way, the antioxidant power lost in the distillation during the deodorization of edible oils has a significant amount of trolls. Until the early 1970s, tocopherols were extracted from these materials and marketed at high prices. But since then, due to the increasing synthesis of surrogate vitamins in the market, the value and importance of these substances as a source for the production of this vitamin has decreased significantly. Synthetic tocopherols are produced from petroleum products.
Vitamin K
This vitamin exists in nature in two forms, K and K2. A synthetic form of this vitamin is also produced, called manadione 17, or vitamin K3, which is about twice as potent as vitamin K (Figure 1). Vitamin K is stable against heat, while it decomposes slowly under the influence of oxygen. Light destroys it quickly. Vitamin K is found in large amounts in food and is synthesized by the intestinal microbial flora. Spinach is the source of this vitamin, and then white cabbage and green peas are other rich sources of this vitamin. Animal products, with the exception of pork liver, are low in vitamin K. Vitamin K plays a role in blood clotting. That's why Henrik Dam, the 18th Danish explorer, called it vitamin K, given that the word coagulation begins in Danish. Vitamin C Vitamin C or ascorbic acid is present in all living tissues and affects oxidation and reduction reactions. This vitamin is a lactone, meaning that inside the molecule, esterification takes place through a reaction between the carboxyl group and the hydroxyl group (Figure 7). Dehydration 19 and taking the carboxyl group 20 can lead to the formation of formalin, which can form polymers and form brown pigments. Give; That's what happens in Mallard's reaction. The human body is unable to absorb ascorbic acid and must be obtained through food. While vegetables and hair contain large amounts of this vitamin, the only animal sources are liver and milk. It should be noted that the only isomeric form is ascorbic acid
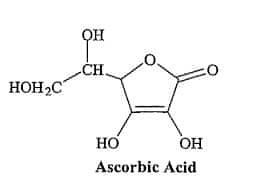
Ascorbic acid
The regenerative properties and ability to form complexes cause the ascorbic acid to react with metal ions. This increases the absorption of these ions from the diet and distributes them throughout the body. This property of ascorbic acid is important in preventing iron deficiency anemia. In this regard, vitamin C is able to counteract the toxic effects of metals. In addition to helping tocopherol to inactivate free radicals formed by oxidation, ascorbic acid itself can do just that. This is important in preventing the adverse effects of ozone and other oxidizing agents on the eye and extracellular fluid. In general, the most obvious physiological role of vitamin is to prevent the occurrence of scurvy 22, which was also discovered in connection with the same disease. Among vitamins, ascorbic acid has the least stability. For example, eating and cutting cucumbers for consumption destroys 78% of the load and consuming 78% of its vitamin C. Destroy the presence of oxygen and heat. These are vitamins. Because ascorbic acid is soluble in water, it is easily removed from food separately with water. But in general, most of its loss is due to chemical reactions. In foods that are especially rich in this acid. (Like fruits), it is destroyed by non-enzymatic browning. In foods such as canned extracts, 23 loss of ascorbic acid occurs twice. In the first step, the destruction is done by combining with the oxygen in the can until the oxygen runs out. The destruction of this acid is in an anaerobic form. It seems that the loss of ascorbic acid in citrus extract powder is only a function of temperature and humidity. Although it seems that even at very low levels of water activity, the decomposition of ascorbic acid occurs, the amount of this destruction is so small that the substance containing this acid can be stored in this condition for a long time. It should be noted that under freezing conditions, even when the temperature is below 1800. C, a significant amount of ascorbic acid may be destroyed. Another factor that destroys this vitamin is S02. Therefore, fruits that are affected by this substance lose some of their vitamin C. Metals such as iron and copper have a specific destructive role on vitamin C. This is why the presence of metal absorbing agents in the food system plays an effective role in maintaining this vitamin. The use of ascorbic acid in the food industry is mainly due to its antioxidant properties Is; There are no restrictions on its use because it is a natural food ingredient. From this point of view, it is also used in the extract of frozen fruits and vegetables Takes. From ascorbic acid in flour products to improve cooking quality and appearance of the product | Final as well as used in the meat processing process. In the latter case, as mentioned in the appendix, ascorbic acid prevents the formation of the carcinogenic nitrosamines in meat. Acorbic fatty acid esters (such as elastic esters) are used to protect oils. Adding ascorbic acid to materials, in addition to enriching it with vitamins, prevents discoloration of food in some processes during storage. It is also used in beverages to remove oxygen and prevent malnutrition. For every inch of oxygen, 3.5 milligrams of ascorbic acid is needed to combine with oxygen to neutralize it. Ascorbic acid is marketed in powder or granular form and is chemically in the form of S-ascorbic acid or its sodium salt as well as A-ascorbic stearate. Ascorbic acid is also used in non-food items such as the advent of photography and in metallurgy as a reducing agent.
Vitamin B (thiamine 2)
This vitamin is involved in the metabolism of carbohydrates and the decarboxylation of alpha-carotids as a coenzyme; However, in this case, it acts in combination with phosphoric acid or thiamine following phosphate, and in this sense it is also called co-carboxylase 27. Good sources of thiamine include whole grains, meat organs such as pears, as well as eggs. Some fish with an enzyme called thiaminease 28 break down thiamine if it is not inactivated before consumption. SO2 and sulfide destroy it. It is believed that the use of these foods in foods that contain significant amounts of this vitamin is not allowed. | Thiamine is one of the most stable vitamins. Its stability depends on the temperature PII, ionic strength and buffer state of the system. From the decomposition of thiamine, a special taste appears, which is similar to the taste that is created during the cooking of meat. As mentioned, sulfite has a specific destructive effect on thiamine. This effect is especially important because sulfites are used to prevent fruits and vegetables from turning brown. In the case of the extinction of thiamine in general, it appears that a rupture is caused by a nuclear-friendly agent at the site where the carbon methane binds the two molecules of the niamine molecule together. That's why a strong nuclear-friendly agent such as HS, sulfite, can easily break down and destroy thiamine. The effect of pH on thiamine destruction occurs under a similar mechanism (Figure 1). Thiamine deficiency causes beriberi, one of the most common symptoms of which is paralysis. To use thiamine in food, its derivatives are used as thiamine
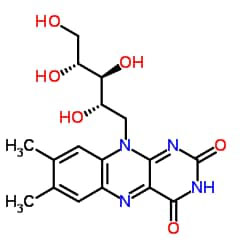
Vitamin B2 (riboflavin)
In nature, this vitamin is usually combined with phosphoric acid and, as a coenzyme - in two forms - participates in some reactions, one of which is flavin mononucleotide 32 (MN) and the other is flavin adenine diucleotide 33 (TAD). . Pyridoxal uses a ATP molecule to form pyridoxal phosphate, which is a coenzyme involved in many reactions related to the conversion and conversion of amino acids, such as the transfer of the amino group to the separation of the carboxyl group. All three forms of this vitamin are resistant to heat but decompose in an alkaline environment. The pyridoxal form of this vitamin is more stable than the others, so it is used to add to some foods and enrich them. This vitamin is widely available in the animal and plant worlds, the richest sources of which are yeast, wheat, meat and tomatoes. As for the destruction of vitamin B6 during food processes, it seems that both its amount and structure will change in the processes of heating, condensing and drying. In the case of milk, boiling it for 2-3 minutes will only destroy about 3% of this vitamin. But sterilizing milk at a temperature of about 120 degrees Celsius for 15 to 13 minutes eliminates 84% of this vitamin. In the thermal method UITST | Extreme heat and short time 4) The rate of destruction is negligible
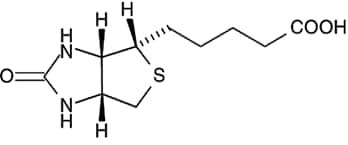
Vitamin B12
This vitamin is chemically more complex than other vitamins Intestinal mucus is released by a free, ready-to-metabolize conjugate 3 enzyme. Basically, folic acid is essentially bound, but in our free-form liver only about 25 percent of the dietary folic acid is freely released. Twice as much as they need to be compared to normal. Folic acid is stable under alkaline and anaerobic conditions. However, the presence of oxygen under the surface is unstable. Also, the solution of this vitamin is under the influence of unstable. D and tetrahydrofolic acid are easily oxidized in air. And it has been shown that when tetrahydrofolic acid is heated to 151 درجه C for 15 minutes in the presence of ascorbic acid, it only loses its activity. But when the level of ascorbic acid in the environment is reduced to 0.5%, no activity of this vitamin remains. Pasteurizing milk by 54HTST (for 2-3 seconds at 92. C) reduces the ability of this vitamin by 12%. But a temperature of 120 degrees Celsius for 15 to 13 minutes destroys 39 percent of this vitamin. In the UHITST method (143 for 3-4 seconds), only 7% of this vitamin was destroyed
Biotin 55
This vitamin has the following structure. There are three asymmetric centers in this building, and moreover, the two connected rings 2 may be cis or trans. Of the eight possible isomeric spaces for vitamins, only cis (+) is naturally and vitamin-active. ا Biotin is widely found in both animal and plant foods Vitamins are freely present in some substances (vegetables, fruits, and milk) and in others (liver, offal, grains, and yeast) in protein. Liver, spinach peanuts, wheat, lettuce, meat, cheese and milk have the highest levels of this vitamin, respectively. As a cofactor, this vitamin is involved in the reactions of carboxylation and trans-carboxylation, and the NI in the ring acts on or transports the carboxyl group. Biotin binds to the enzymes it needs by forming an amide bond between the ampicillin lysine enzyme and its carboxyl group. It should be noted that biotin is completely resistant to heat, light, oxygen in the air, and environments that are poorly acidic or neutral (PH 5-8). Nitric acid destroys the biological activity of this vitamin. Formic aldehyde also destroys biotin. In general, according to studies, this vitamin is very stable in industrial or commercial processes of food with the operation that takes place at home on food. As mentioned in the topic of proteins, in egg whites, biotin is strongly attached to avidin. As avidin is denatured by heat, the bad effect of this protein is eliminated from biotin. Deficiency of this vitamin in humans seems unlikely. Because, as mentioned, biotin is widely available in various foods and is now produced by intestinal bacteria. It is important to note that the amount of biotin excreted in the urine is the amount of vitamin B1 that enters the body, which indicates the large role of intestinal bacteria in biotin. That's why you can't recommend a certain amount of this vitamin every day. 5. Minerals 59 Minerals in food may be in the form of mineral or organic salts or in combination with other organic substances, such as phosphorus in phospho proteins or metals in enzymes. And the amount of minerals in food varies greatly from one substance to another, depending on environmental factors such as soil composition for the plant and the nature of the nutrient fed to the animal. Mineral loss due to chemical reactions is less than the destruction of these materials by mechanical methods such as peeling. An important part of the destruction of minerals is due to their solubility in water. Some minerals react with other food components. For example, oxalate and phytate multivalent anions can form salts with bivalent cations, which are very insoluble. In this sense, they pass through the gastrointestinal tract without being absorbed. Therefore, the issue of mineral availability in the digestive system is very important. Among anions, only fluoride, iodine, and phosphate are of particular nutritional importance. Many metal ions can be attached to organic molecules in the form of ligand 57. Examples include iron, copper in cytochromes, magnesium in chlorophyll, and cobalt in vitamin B12. It should be noted that the amount of a mineral in different products of a food is absorbed by iron, which should probably be due to the presence of phytate in it. The availability and usability of the gastrointestinal tract is also dependent on external factors and internal factors of the consumer's oven. In general, in food planning, special and necessary attention should be paid to the subject that can be used on food components in the body. The following is a brief overview of the status and critique of important minerals in human nutrition. These can be divided into two groups: major minerals and minerals or trace elements. ا Major minerals are at the top of this group of sodium, which is 1.4 grams per kilogram of body weight. It is located outside the cell and regulates the osmotic pressure of extracellular fluid. In addition, it plays an important role in the activity of some enzymes such as amylase. The amount of sodium intake in the daily diet of 1.7 to 6.9 grams varies. The recommended amount for adults is one gram per day (in the form of sodium chloride). Low sodium intake or high sodium intake can lead to serious deficiencies in the body. Excessive consumption causes high blood pressure. Naturally, salty foods such as salted fish are the richest food source in this regard. The amount of sodium in meat, milk, eggs, spinach and celery is moderate, but low in fruits and other vegetables. Another important element is potassium, which is 2 grams per kilogram of body weight. Potassium regulates the osmotic pressure inside the cell. It is also involved in the transport and passage of substances through cell membranes and is effective in activating some glycolytic and respiratory enzymes. Lentils, potatoes and nuts are rich sources of potassium and butter and honey are very poor sources. The recommended dose is one gram per day (in diets low in sodium). It is one of the most important building blocks of the body. In addition to its structural role, calcium is important in remembering blood and muscle contraction. The main source of calcium for the body,. And its products, and other foods, whether animal, such as egg meat, or sometimes vegetables, fruits, and grains, have far less calcium. The recommended amount is 1-0 per day / 4 grams. | The amount of chlorine in body tissues is 1.1 grams per kilogram of body weight, which is basically fed to the body through salt. Chlorine acts as an anti-sodium ion in extracellular fluids and also acts against hydrogen ions in gastric juice. The total amount of phosphorus in the body is about 700 grams. The daily requirement is 0.1 - 0.8 g. The calcium-phosphorus ratio in food should be about one. The ratio of calcium to phosphorus is 2 to 1 in bone, 1.2 to 1 in cow's milk, and 2 to 1 in human milk. It is recommended that this ratio be 1.5 to 1 at the beginning of the baby's life and 1.2 to 1 after a while, and that this ratio be 1 to 1 for adults. Some types of cheese and lentils are rich sources of phosphorus. The amount of phosphorus in meat, milk, bread and vegetables is moderate. And the amount of magnesium in the body is 250 mg per kilogram of body weight. It acts as a component or activator of some enzymes, especially those involved in the conversion of strong phosphate bonds (for example, in the glycolysis process). Cocoa, soy and almonds are good sources of this element, but meat and milk are poor sources in this regard. The recommended daily allowance of magnesium is 0.3 g. The basic essential elements of iron in the body are 4-5 grams, most of which are combined with hemoglobin and myoglobin. Iron is also associated with some enzymes, such as peroxidase, catalase, and hydrolase, which is an essential component of the daily diet. The amount of iron needed depends on one's age and gender. But in general, this requirement is 2.8-1 mg per day. Different parts of it can be different. For example, in terms of chromium content, there is a big difference between dairy products and such a difference between egg yolk and egg white. As mentioned earlier, solubility in water is one of the reasons for the loss of minerals, so de-enzymatic action can be important in this regard. It shows the loss of minerals in spinach during this process. Of course, losing nitrate is good. Because it is good in terms of providing and maintaining health, as well as reducing the corrosive effects of food on its processing systems. Measuring the elements in the food consumed is the only weak indicator in terms of nutrition. Because what is important here is the availability or usability of the elements in the gastrointestinal tract that are affected by various factors. The type of food affects the usability of iron in the digestive tract. In this case, animal foods are superior to plant foods and specifically some grains. Of course, other factors also play a role. For example, vitamin C increases iron absorption. Phosphate, and to a lesser extent calcium, reduces iron absorption. Whole grains also cause a decrease in the production of the very sweet substance naringin dehydrocalcon, which is about the sweetest sucrose
References
Food Chemistry, Dr. Hassan Fatemi
Desrosier, N. W. (ed.). 1977. Elements of Food Technology. AVI, Westport, Duckworth, R. B. (ed.). 1975. Water Relations of Foods. Academic Press, London. 12. Eskin, N. A. M. 1990. Biochemistry of Foods, 2nd edn. Academic Press, London. 14. FAO, WHO and UNU Expert Consultation. 1985. Energy and Protein Requirements. Technical report Series 724. World Health Organization, Geneva. 15 - Fellows, P. J. 1988. Food Processing Technology: Principles and Practice. Ellis Horwood, New York. 16 - Fennema, O. R. (cd.). 1976. Principles of Food Science: Part I, Food Chemistry. Marcel Dekker, New York. 17 - Fennema, O. R. (ed.). 1985. Food Chemistry, 2nd edn. Marcel Dekker, New York. 18 - Fennema, O. R. (ed.). 1996. Food Chemistry, 3rd edn. Marcel Dekker, New York. 19 - Fox, P. F., Morrissey, P. A. and Mulvihill, D. M. 1982. Chemical and enzymatic modification of food Proteins. In Developments in Food Proteins, ed. Hudson, B.J. F., Applied Science Publishers, London. 20 - Furia, T. F. (ed.). 1972. Handbook of Food Additives, 2nd edn. CRC Press, Boca Raton, Florida. 21 - Giese, J. H. 1993. Alternative Sweeteners and Bulking Agents. Food Technol. 47(1), 114-126. 22 - Giese, J. H. 1995. Vitamin and Mineral Fortification of Foods. Food Technol. 49(5), 110-122. 23 - Gunstone, F. D. and Norris, F. A. 1983. Lipids in Foods: chemistry, biochemistry and technology. Pergamon Press, Oxford. 24 - Hammond, E. G. 1985. Stability of soybean oil to oxidation. In Proceedings of the World Soybean Research Conference III, ed. R. Shibles, Westview Press, London. 25 - Harris, P. (ed.). 1990. Food Gels. Elsevier Applied Science, London. 26 . Harris, R. S. and Karmas, E. (eds.). 1977. Nutritional Evaluation of Food Processing, 2nd edn. AVI, Westport, Connecticut. 27 - Health, H. B. and Reineccius, G. 1986. Flavor Chemistry and Technology. AVI, Westport, Connecticut. 28 . Heckman, E. 1977. Starch and its modifications for the food industry. In Food Colloids, ed. Graham, H. D., AVI, Westport, Connecticut. 1. Alias, C. and Linden, G. 1991. Food Biochemistry, Ellis Horwood, New York 2. Anon. 1998. INFORM. 9(2), 175. 3 - Aurand, L. W. and Woods, E. A. 1973. Food Chemistry. AVI, Wem Pon, Connecticut 4. Baianu, I. C. (ed.). 1992. Physical Chemistry of Food Procemes, Vol. I, Van Nostrand Reinhold, New York, 5. Belitz, H. D. and Grosch, W. 1987. Food Chemistry, Springer Verlag, Bertio 6 - Bennink, M. R. and Srisuma, N. 1989. Digestibility of dry legume Starch and Protein. In Proceedings of the World Congress on Vegetable Protein Utilization in Human Foods and Animal Foodstuffs, ed. Applewhite, T. P., AOCS Press, Champaign, Illinois. 7. Berk, Z. 1976. Introduction to Biochemistry of Foods. Elsevier Scientific Publishing Company, Amesterdam. 8. Charly, II. 1970. Food Science. The Ronald Press Company New York. 9. Coultate, T. P. 1996. Food-The Chemistry of Its Components, 3rd edn. Royal Society of Chemistry, Cambridge. 10. deMAN, J. M. 1990. Principles of Food Chemistry, 2nd edn. Van Nostram Keinhold, New York
- Service ProvidersBandar Food Industry Knowledge Group
- DateJune 30 2020
- Grouping News and Articles
- Source linkwww.BandrFood.ir
- Subscribe




