
Water
Basically, water is an important part of many foods. In addition, water has special and unique properties that create special conditions and functions in food systems. Awareness of these features is very important, especially in the food industry
Water building
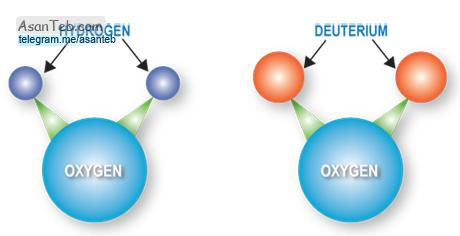
The water molecule consists of two hydrogen atoms and one oxygen atom. However, the position of hydrogen atoms relative to oxygen is such that these atoms form an angle of 105 degrees with each other. In this sense, the water molecule is not electrically balanced, that is, there is a negative charge on the oxygen atom and some positive charge on the side where the hydrogen atoms are located. The result is that the water molecule is a polar compound, and in relation to polar and non-polar materials, it exhibits a function commensurate with this property. Undoubtedly, this polarity also affects water molecules themselves, linking them to bonds called hydrogen bonds. A water molecule can be thought of as the center of a regular quadrilateral. In this case, according to Figure 1, oxygen can bond with two water molecules and each hydrogen with one water molecule. In this way, all five water molecules come together to form water-specific properties. To pay more attention to these properties of water, it is sufficient to compare water with substances that have similar molecules - such as ammonia and methane. For example, while water is normally liquid and has a high evaporation point of 100 degrees Celsius, wind compounds Chemical food chemistry is steadily declining. However, by lowering the water temperature from 4 to the line of reason for the formation of different types of hydrogen bonds between the molecules, some water volume is added. As the water freezes, the volume increases dramatically. In this case, the existing hydrogen bonds are destroyed and new bonds are formed that connect all four water molecules. Hydrogen bonds are also destroyed during water evaporation. In addition to heat, ions (or ionizers) as well as compounds that have the ability to form hydrogen bonds affect the natural structure of water and its bonds, altering them and forming new forms of bonds. Because of this change, if there are such substances and compounds in the water, the cooling of the water must be done below zero degrees Celsius to freeze it. Mineral ions that, for example, are obtained from the decomposition of salt, are unable to form hydrogen bonds and only absorb a certain part of the water molecule with the help of their load type. Although in saline dilute solutions only the water layers around the ion undergo structural change and the main mass of water remains unchanged, in concentrated salt solutions the structure of this water also undergoes changes. According to the available evidence, some ions change the structure of water in such a way that the resulting solution has a lower fluidity than pure water. Ions with strong electric fields such as sodium, calcium and aluminum can cause this. But another group of ions increasesThe fluidity melts. Ions with weak electric fields such as potassium, ammonium and chlorine are part of this group. These ions, while altering the original structure of water, unlike the ions of the first group are not able to provide a strong new structure for it.
۲ - نمودار فازهای آب ۲
The water molecule consists of two hydrogen atoms and one oxygen atom. However, the position of hydrogen atoms relative to oxygen is such that these atoms form an angle of 105 degrees with each other. In this sense, the water molecule is not electrically balanced, that is, there is a negative charge on the oxygen atom and some positive charge on the side where the hydrogen atoms are located. The result is that the water molecule is a polar compound, and in relation to polar and non-polar materials, it exhibits a function commensurate with this property. Undoubtedly, this polarity also affects water molecules themselves, linking them to bonds called hydrogen bonds. A water molecule can be thought of as the center of a regular quadrilateral. In this case, according to Figure 1, oxygen can bond with two water molecules and each hydrogen with one water molecule. In this way, all five water molecules come together to form water-specific properties. To pay more attention to these properties of water, it is sufficient to compare water with substances that have similar molecules - such as ammonia and methane. For example, while water is normally liquid and has a high evaporation point of 100 degrees Celsius, wind compounds Chemical food chemistry is steadily declining. However, by lowering the water temperature from 4 to the line of reason for the formation of different types of hydrogen bonds between the molecules, some water volume is added. As the water freezes, the volume increases dramatically. In this case, the existing hydrogen bonds are destroyed and new bonds are formed that connect all four water molecules. Hydrogen bonds are also destroyed during water evaporation. In addition to heat, ions (or ionizers) as well as compounds that have the ability to form hydrogen bonds affect the natural structure of water and its bonds, altering them and forming new forms of bonds. Because of this change, if there are such substances and compounds in the water, the cooling of the water must be done below zero degrees Celsius to freeze it. Mineral ions that, for example, are obtained from the decomposition of salt, are unable to form hydrogen bonds and only absorb a certain part of the water molecule with the help of their load type. Although in saline dilute solutions only the water layers around the ion undergo structural change and the main mass of water remains unchanged, in concentrated salt solutions the structure of this water also undergoes changes. According to the available evidence, some ions change the structure of water in such a way that the resulting solution has a lower fluidity than pure water. Ions with strong electric fields such as sodium, calcium and aluminum can cause this. But another group of ions increasesThe fluidity melts. Ions with weak electric fields such as potassium, ammonium and chlorine are part of this group. These ions, while altering the original structure of water, unlike the ions of the first group are not able to provide a strong new structure for it.
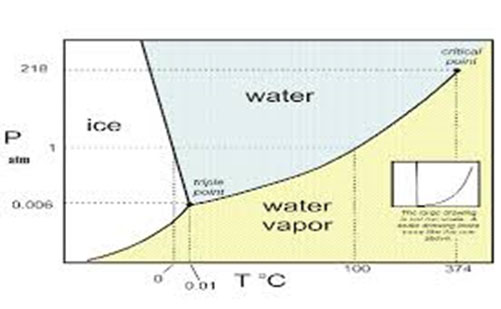
- Water phase diagrams 2
The relationship between different water states, such as liquid, solid, and vapor, is very important in many food processes, such as condensing, drying, freezing, drying in freezing, and cooling under the line. This relationship can be distinguished from the water phase diagram.
B - Published layer
This layer has less bonding strength and is weaker attached to the absorption layer. This layer is not yet free water. In fact, this layer is not uniform, and the farther away it is from the absorption layer, the more real the water takes shape. Of course, changing the water state of all its layers has no clear boundaries. The thickness of the absorption layer is about 2 angstroms and the thickness of the diffused layer is up to 800 angstroms
C - free water layer
This water has all the characteristics of ordinary water. Tissues that have a large amount of water attached are less likely to be damaged during freezing than tissues that have lower amounts of this water. In addition, this water has a special effect on freezing reactions and is an important factor in preventing the formation of crystals. The amount of non-freezing water (depending on the amount of protein present that plays an important role in water absorption) varies from one substance to another. For animal tissues, 8-10% is recommended; In some vegetables it is less than 1% and in whole corn it is 34%.

- Water activity 10
Corruption of food during storage in the warehouse can be done quickly by microorganisms, while enzymatic and chemical reactions take place more slowly. In both cases, water is the most important factor in controlling these benefits. The water content of a food may be reported based on its wet weight or dry weight. From the point of view of calculation for processes, dry weight is considered more, while in food tables, the wet weight of food is listed and considered. Of course, the mere amount of water in a food item cannot be used to measure corruption. Because, for example, peanut oil spoils more than 0.9% of moisture; If the potato starch is still stable at 20% humidity. Therefore, the issue of water existence or availability will be discussed here, which is considered as water activity (e). Some processes affect the existence of moisture by removing moisture from food, such as drying, freezing drying, evaporation, and condensation through freezing. But others do it by immobilizing or stabilizing 11 waters. These include the use of moisture-absorbing substances such as invert sugar 13 (in medium-moisture foods 14) and the conversion of water food chemicals into ice crystals through freezing. So, in short, there are two types of processes, one is the removal of water and the other is the consolidation of water.
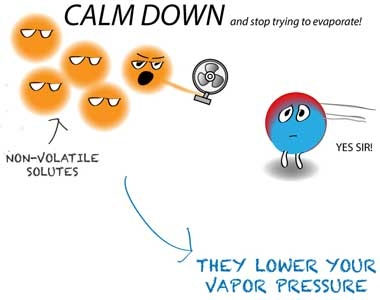
Water in food has a vapor pressure that depends on the amount of water: A- The amount of water available, 2- Temperature and 3- The concentration of soluble substances (especially salts and sugars) in water According to the definition, water activity is The ratio of water vapor pressure in food to the saturated vapor pressure of water occurs at the same temperature of the material in which the amount of water activity is more than 0.3-0.2. The closed curve thus formed is called the heterocycle cycle. The cause of hysteria can be the following: A. During the separation of water from the nutrient, interactions take place between its solid components, so that some of the water-absorbing points are lost
- There is a difference in water vapor pressure required to fill and empty the capillaries with an irregular structure of water. Due to the fact that in the same amount of water activity, food samples have less water during reabsorption than when water is lost, their oviposition will be higher. One of the consequences of such a situation could be that in practice Reabsorption of water is less oxidation than water loss (in a given water activity). In general, materials that absorb moisture more easily have a lower temperature than other absorption curve materials. In the case of many foods, the first part of the absorption curve is completely flat, which indicates that the food has a low tendency to absorb water. But the next part has a completely sloping shape curve, which shows the ease of moisture absorption in this part. Such curves are specific to those foods that are high in sugar or salt and have little water absorption in their hair follicles. Starchy substances such as wheat and rice absorb more water in low water activity than protein substances such as meat. In general, sugars as a single group show two types of temperature absorption curves. In the amorphous or amorphous state, the nia easily absorbs moisture. If in the crystal state such a property is shown to a small extent. Here a problem can occur due to the change from stateless to crystalline, which is associated with the release of water. In such a case, if Nand is part of a food system, then other substances in the cinema absorb water, which is usually accompanied by chemical and physical changes. The operational effect, such as heating the food on absorption behavior and, of course, the shape of the absorption curve, can be greater than when a substance with less or more water absorption is added to it. In general, due to heat, the water absorption capacity of protein materials decreases. Because their active vitality in such a process decreases due to denaturation Other operations such as desalination and PII alteration have similar effects). Covering the surface of the moisture-absorbing material with grease also reduces the number of active points. The use of moisture-absorbing substances in food causes the amount of water activity in them to decrease without reducing water. Substances such as salt, sugar, glycerol and propylene glycol 25 can do this. These materials are especially used for foods that have a moisture content of 35-15% and water activity, as well as substances that need to be stored for a long time (such as yeast). In general, the action of water absorption and its effect on the physical properties of food can be in three ways: 1- Absorption without structural change in the adsorbent, such as adsorption by sugar crystals 2 - Absorption with structural change in the adsorbent such as milk and egg white. 2- Absorption along with the formation of a solution such as sugar solutions. Water activity and food stability Almost all microbial activities in less than 0.0 They stop. This figure is less than 0.7 for most cakes, less than 0.8 for yeasts, and less than 0.9 for bacteria. The interaction of water activity with temperature, PII, oxygen, carbon dioxide or chemical preservatives has an important role in preventing the growth of microbes. If one of the environmental conditions (other than water activity) is not optimal for a particular microorganism, then the effect Reducing the amount of water activity on our microorganisms increases. This allows the combination of several gentle control mechanisms to preserve the food so that it does not cause a significant reduction in its sensory and nutritional properties. Enzymatic activity actually stops at values below the BET single-layer value. This is due to the low mobility of the substrate and its inability to penetrate the active site on the enzyme. Chemical changes, They are more complex. Two of the most common low-calorie foods are Millard browning and fat oxidation. The amount that causes the maximum amount of browning in food depends on the type of food. But in general, at the bottom, the mobility of the substances that enter the reaction is low, which leads to a decrease in browning. Usually the maximum browning in iTin 0 activity / 6, is done. From the point of view of food groups, materials with moderate humidity (moisture content of 40-20%), cake replacement, dates and banner banners show the maximum of this reaction. In high humidity levels, due to the reaction of the reactants, the browning of the reduction acts as a deterrent to the continuation of the reaction. Oxidation of fats occurs in small amounts due to the action of free radicals. In addition to the amount of single-layer BET water, antioxidants and metal-forming agents dissolve and reduce the amount of oxidation. To a greater extent, the catalytic activity of metals decreases due to the absorption of water and the formation of insoluble hydroxides. However, in large quantities, it is dissolved with metal catalysts and the structure of the food becomes swollen. Figure 4 shows the effect of water activity on the factors that cause spoilage and adverse changes in food. Most enzymes in water activity are less than 85 /. They are ineffective. But lasers can be active up to 28 to 0.3 or even 0.1 water activity. Separation of the enzyme from the substrate largely prevents such adverse reactions. If the substrate is liquid, it will be affected by the enzyme much faster. For example, the hydrolysis of liquid oil in water activity is observed to be equal to 0.15, while solid oil in such conditions is slightly hydrolyzed
Fat oxidation is as complex as the amount of water activity mentioned earlier. It seems that keeping the dried and frozen materials in a freezing state above the amount of single-layer moisture that covers the surface of the components will provide maximum protection against oxidation. Studies on oxidation in frozen salmon have shown that fat oxidation is reduced by increasing the amount of fish water. Of course, in the case of foods such as various ingredients and types of reactions can be discussed, the total amount of water activity (or percentage of moisture required to obtain the least adverse reactions should be obtained).

The components in a certain amount of moisture have already been balanced with their surroundings. Placing these components inside a single package without exchanging them with the outside environment causes moisture exchange between them, which may be harmful to one component or more and reduce the quality or even spoilage of the material inside the package. . In this regard, before placing such components in a single package, it is necessary to adjust and adjust their humidity level to a suitable level so that this problem does not arise later.
References
Food Chemistry, Dr. Hassan Fatemi
Desrosier, N. W. (ed.). 1977. Elements of Food Technology. AVI, Westport, Duckworth, R. B. (ed.). 1975. Water Relations of Foods. Academic Press, London. 12. Eskin, N. A. M. 1990. Biochemistry of Foods, 2nd edn. Academic Press, London. 14. FAO, WHO and UNU Expert Consultation. 1985. Energy and Protein Requirements. Technical report Series 724. World Health Organization, Geneva. 15 - Fellows, P. J. 1988. Food Processing Technology: Principles and Practice. Ellis Horwood, New York. 16 - Fennema, O. R. (cd.). 1976. Principles of Food Science: Part I, Food Chemistry. Marcel Dekker, New York. 17 - Fennema, O. R. (ed.). 1985. Food Chemistry, 2nd edn. Marcel Dekker, New York. 18 - Fennema, O. R. (ed.). 1996. Food Chemistry, 3rd edn. Marcel Dekker, New York. 19 - Fox, P. F., Morrissey, P. A. and Mulvihill, D. M. 1982. Chemical and enzymatic modification of food Proteins. In Developments in Food Proteins, ed. Hudson, B.J. F., Applied Science Publishers, London. 20 - Furia, T. F. (ed.). 1972. Handbook of Food Additives, 2nd edn. CRC Press, Boca Raton, Florida. 21 - Giese, J. H. 1993. Alternative Sweeteners and Bulking Agents. Food Technol. 47(1), 114-126. 22 - Giese, J. H. 1995. Vitamin and Mineral Fortification of Foods. Food Technol. 49(5), 110-122. 23 - Gunstone, F. D. and Norris, F. A. 1983. Lipids in Foods: chemistry, biochemistry and technology. Pergamon Press, Oxford. 24 - Hammond, E. G. 1985. Stability of soybean oil to oxidation. In Proceedings of the World Soybean Research Conference III, ed. R. Shibles, Westview Press, London. 25 - Harris, P. (ed.). 1990. Food Gels. Elsevier Applied Science, London. 26 . Harris, R. S. and Karmas, E. (eds.). 1977. Nutritional Evaluation of Food Processing, 2nd edn. AVI, Westport, Connecticut. 27 - Health, H. B. and Reineccius, G. 1986. Flavor Chemistry and Technology. AVI, Westport, Connecticut. 28 . Heckman, E. 1977. Starch and its modifications for the food industry. In Food Colloids, ed. Graham, H. D., AVI, Westport, Connecticut. 1. Alias, C. and Linden, G. 1991. Food Biochemistry, Ellis Horwood, New York 2. Anon. 1998. INFORM. 9(2), 175. 3 - Aurand, L. W. and Woods, E. A. 1973. Food Chemistry. AVI, Wem Pon, Connecticut 4. Baianu, I. C. (ed.). 1992. Physical Chemistry of Food Procemes, Vol. I, Van Nostrand Reinhold, New York, 5. Belitz, H. D. and Grosch, W. 1987. Food Chemistry, Springer Verlag, Bertio 6 - Bennink, M. R. and Srisuma, N. 1989. Digestibility of dry legume Starch and Protein. In Proceedings of the World Congress on Vegetable Protein Utilization in Human Foods and Animal Foodstuffs, ed. Applewhite, T. P., AOCS Press, Champaign, Illinois. 7. Berk, Z. 1976. Introduction to Biochemistry of Foods. Elsevier Scientific Publishing Company, Amesterdam. 8. Charly, II. 1970. Food Science. The Ronald Press Company New York. 9. Coultate, T. P. 1996. Food-The Chemistry of Its Components, 3rd edn. Royal Society of Chemistry, Cambridge. 10. deMAN, J. M. 1990. Principles of Food Chemistry, 2nd edn. Van Nostram Keinhold, New York
- Service ProvidersBandar Food Industry Knowledge Group
- DateJune 30 2020
- Grouping News and Articles
- Source linkwww.BandrFood..ir
- Subscribe




