Proteins
1- Proteins
Proteins are polymers of amino acids that, due to their structure and chemical composition, are important in the physical and tissue properties of food and in creating flavor in food. Biologically, they help the body make proteins and enzymes and many hormones by providing essential amino acids.
1.1 - Amino acids
Although there are many amino acids in nature, only 20 of them are involved in protein building, the structural form of amino acids is shown below. The R side chain determines the type of amino acid, and the unique characteristics of each amino acid are related to this chain. Depending on the type of side chain, amino acids are divided into 4 groups:
1 - Amino acids with non-polar and non-pregnant chains such as: alanine, leucine, isoleucine, methionine, phenylalanine, proline, and valine
2 - Amino acids with polar and non-pregnant side chains such as: serine, threonine, tyrosine and cysteine. These amino acids can bind to other amino acids through hydrogen bonding.
3 - Amino acids with a positive side chain with positive openings such as lysine, arginine and histidine
4 - Amino acids with a negative side chain such as glutamic acid and aspartic acid
Another type of amino acid classification is biologically, in which amino acids are divided into two categories, essential and non-essential. Essential amino acids are not made in the body and must be obtained through a diet that includes: valine, leucine, isoleucine, threonine, phenylalanine, tryptophan, methionine and lysine. Histidine is also essential for humans and arginine for animals. Natural amino acids are L in terms of spatial structure. All amino acids except glycine, the R-group of hydrogen, contain asymmetric carbon, so they deflect polarized light and are so-called optical.
Amino acids, due to having carboxylic and amine groups, can exist in terms of pH in the form of cations, anions, or bisexual ions.
Amino acids in an acidic environment are high concentrations of hydrogen ions (as cations, and in alkaline environments as anions.) This pH is called the isoelectric point (PI). It has bisexual ions (amino acids with one carboxyl group and one PI 5-6 amine group).
1.2 - Proteins
From the interaction of the amine group with an amino acid with the carboxyl amino acid group, a bond is formed before it, which is called a peptide bond. The result of this reaction is a dipeptide. With the accumulation of more amino acids by peptide bonds, oligopeptide and then polypeptide are formed. When the number of amino acids in a polypeptide chain is more than 100, it is called a protein.
Proteins are divided into simple and compound groups. Simple proteins are proteins that are made up of only amino acids and only amino acids are obtained by their decomposition. Lipoproteins (be.
Proteins can also be classified according to their solubility. In this regard, proteins include:
Albumin: A water-soluble and heat-sensitive solution of globulin: Soluble in weak salt solvents Glutelins: Soluble in 70% alcohol Glutelins: Soluble in acidic solutions and dilute play. It also contains prolamins, scleroproteins, proteins and histidines.
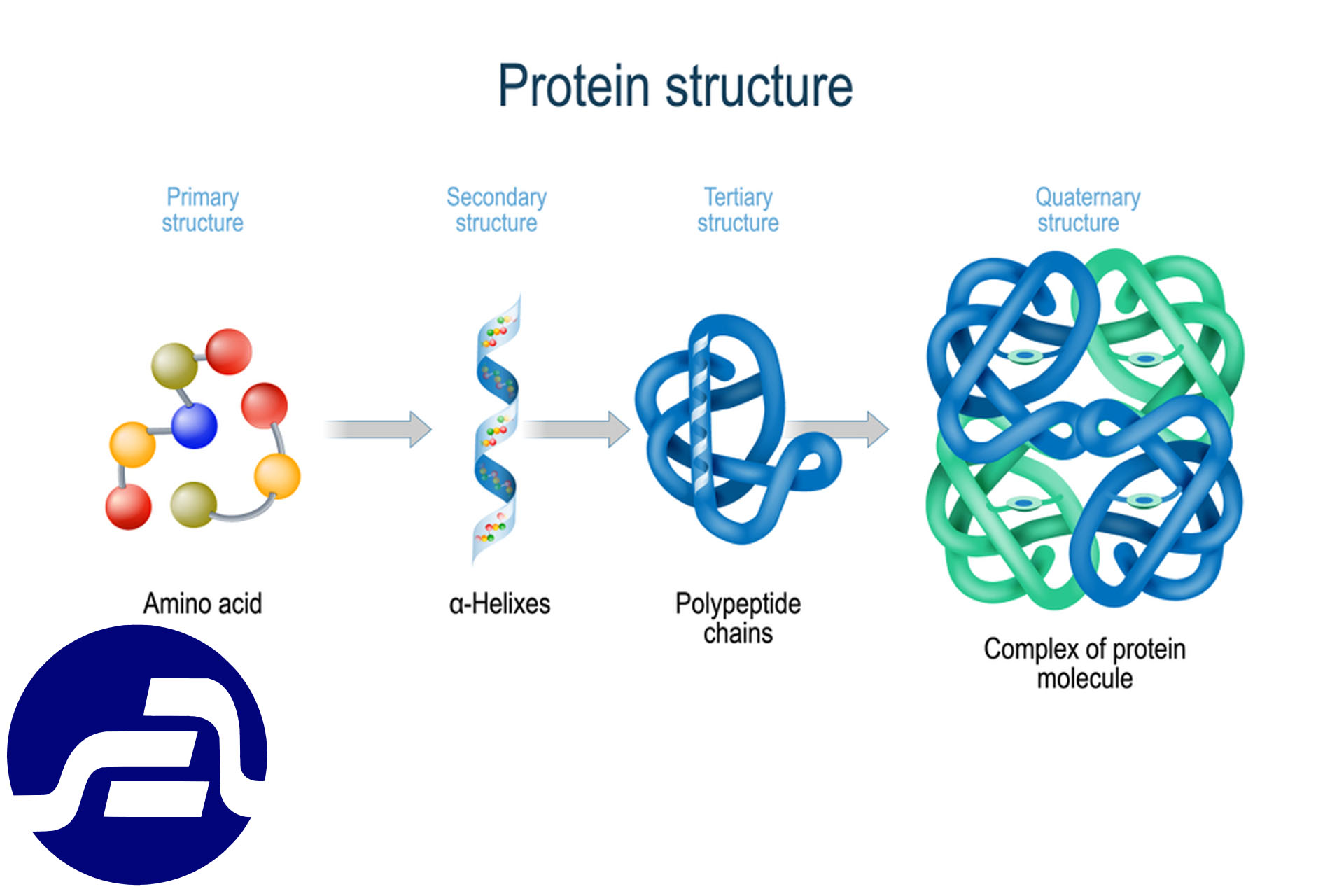
1.2.1 - Protein structure
Building Type I: This building is made up of amino acids that come together and actually determine the order in which the amino acids are placed. The first type of building also determines the next protein structure. To write the order of the amino acids, use the three letters of the first name of each amino acid, and the order of the amino acids is written in such a way that the amino acid at the end of N) is amine (on the left side).
Val - Pro - Ala
In this peptide chain shown, valine is val (amino acid end N). Ala is also amino acid end C) carboxyl (.
The N-end amino acid can be determined by the Sangar reaction or the use of the aminopeptidase enzyme. The C-terminal amino acid can also be determined by the protein reaction with hydrazine or the effect of the carboxypeptidase enzyme that separates the C-terminal amino acid.
The most complete method for determining the sequence of amino acids is the method of adenoma decomposition. In this method, the amino acid N at the end of the protein reacts with phenyl isothiocyanate and peptides the production of N-phenyl thiocabamyl. The complex is then broken down by hydrochloric acid and the end-amino acid N is isolated as a circular compound that is detected by special methods.
The peptide bond is trans. This bond has a resonant state (NH - CO (and therefore not a single single bond. This bond has features between a single and double bond. Therefore, space rotation, unlike other single bonds, is not possible for this bond. Adjacent to the peptide bond) α carbon, which is located on both sides of this bond (it can rotate and with its rotations determine the spatial shape of the protein. In these rotations, the type and shape of the R side chain play a decisive role in the type of rotation.
Second Building: The second building of the protein is usually in the form of a helix or folded sheet. Among the different types of helixes, the alpha type has the highest frequency. In this spiral, there are 3.6 amino acids per round and the direction of rotation in natural amino acids is L type from right to left and from top to bottom. The side chains are also located outside the ring. During the oxygen spiral connected to the peptide carbon) the carbon that formed the peptide bond (the amino acid n bonded to the nitrogen attached to the peptide carbon n + 4 hydrogen.
Hydroxyproline Because amino acids in these amino acids are attached to peptide carbon without hydrogen, the reason is that the amine group is in the ring (so it cannot stabilize the spiral by creating the hydrogen bond mentioned above. Therefore, proteins) It has high levels of proline acids and hydroxyprolines. Immunosuppressants (do not form an alpha helix.) Because protein lacks a spiral structure, it has high levels of these two amino acids.
Also, the presence of amino acids with the same charge has a negative effect on the formation of the helix due to the creation of repulsive force and also the presence of the amino acid isoleucine due to the high spatial barrier of its side chain.
In the shape of the sheets of the second protein structure, the angle between the alpha carbon and the C-N bond is greater and the molecule is stretched and zigzagged. Due to this more elongated structure, it is not possible to form hydrogen bonds like what was said in the alpha helix. In this case, the side chains are placed at the top and bottom of the page. The peripheral chain of the amino acids of each plate establishes a hydrogen bond with the side chains of the adjacent amino acids of the plate. If the two chains that bind the hydrogen bond are the same (for example, both from the end). N is towards C (the structure created is called parallel and otherwise it is called non-parallel.
Third Building: This building is created by folding the protein chain along the axis of the chain and placing each part of the chain next to the other parts of the chain. Side chains are created. Hydrophobic hydrophobic interventions (also involved in the stability of the molecule and by the accumulation of hydrophobic parts inside and hydrophilic parts) hydrophilic (externally formed). Of course, this is not always the case; for example, in insoluble proteins such as fat-carrying lipoproteins. There are a lot of protein-rich amino acids in the blood.
Fourth Building: It is created by connecting two or more peptide chains with non-covalent bonds. Each of these chains is called a subunit. Hemoglobin) includes 2 alpha subunits and 2 folded beta subunits (myosin with 6 subunits and some enzymes). In addition to the peptide bonds that make up the first structure of the protein, other interventions involved in protein stability include:
Hydrogen bonding: Its energy is 2-10 kcal per mole.
Disulfide bonds: Similar to peptide bonds, they are covalent and form between the sulfhydryl groups (SH) and bind to different parts of the protein, resulting in a circular structure in the protein structure. The energy of this bond is 80 kcal. It is on the mole.
Hydrophobic interactions: It has 1-3 kcal of energy per mole. If the side chains of non-polar amino acids are in the polar solvent, these chains try to move away from the environment and accumulate together, which is called hydrophobic interactions.
Factors affecting gelatinization and the properties of the resulting dough
High concentrations of sugar in the environment reduce the amount of gelatinization, reduce maximum viscosity and reduce the strength of the gel. In this regard, the effect of disaccharides is greater than monosaccharides. The effect of each sugar in reducing the amount of gelatinization and reducing the viscosity and strength of the gel are:
Sucrose> lactose> glucose> fructose
Suckers, which are associated with bodybuilding, reduce the viscosity and excessive stiffness of the pudding. However, low concentrations of sugar can increase the viscosity of gelatinous starch.
Fats and fatty acids, menus, di, and triglycerides, by forming a complex with emylose, on the one hand, resist water penetration into granulated water, and on the other hand, make it difficult to remove complexed amylose, thus causing a negative effect on gelatinization, reducing Increases viscosity and gel formation. Also, fat-emulsion complexes simply cannot participate in the bonding between the granules needed to make the gel.
Very low pH due to the breakdown of starch granules and converters into small pieces) is more effective on amylopectin (such as dextrins that have no concentrating effect, reducing the maximum viscosity of starch.
To prevent this effect, it is possible to use starch molecules connected to coarse starch molecules so that hydrolysis does not have much effect on them.
In the early stages of starchy, the bonds in the emulsion chains are responsible for creating starch in the bread. Fats and emulsifying substances can prevent the amylose strings from joining together. The stagnation caused by long-term storage of bread is due to the joining of the amylopectin strands. Heat by breaking These bonds and the re-pouring of water between the strands of amylopectin to some extent restore the bread to its original state. Freezing at low temperatures) Refrigerator temperature (occurs faster than high temperature. Freezing completely prevents stagnation.
Cereal starches such as corn, wheat and rice, after cooling, form a firm gel and the color is opaque. Stem stems and roots, on the other hand, form apple and cassava (clear but weak gels). Pre-gelatinized starches absorb water faster and do not need high temperatures to create viscosity.
Modified Starch: Modified :(
By using acid to hydrolyze starch, the acid hydrolyzes parts of the starch, which is mostly amylopectin, and parts of the crystal, which is mainly emylose, remain intact. In this case, the starch produces low viscosity and requires a high temperature to increase the viscosity) because the ratio of emylose to its amylopectin is increased (this starch is suitable for use in canned foods). Another type of modified starch is oxidized starch with sodium hypochlorite (which has low viscosity and is transparent. The most important modified starches are phosphate starches. When monophosphate starch is added to food, it reduces water supply during defrosting. Monophosphate starches are also soluble in cold water. In another type of phosphate starch, a phosphate group forms a bond between two hydroxyl agents, mainly belonging to two different starch chains. This type of starch is called attached starch. These starches are less swollen than ordinary starches and are resistant to disruption and hydrolysis. Also, these starches have a very high gelatinization temperature.
4.4 - Glycogen
Carbohydrates are stored in animal tissues and are present in small amounts in the liver and muscles. It is structurally similar to amylopectin but has a higher molecular weight and number of branches. In glycogen, after every three units of glucose, there is a 6-7 unit branch of glucose. [08:58, 01/07/2020] Tanaz: .4.3 - Corn syrup production Corn syrup is one of the starch products that is widely used in the food industry. The process may be performed by acid or by acid and enzymes. The progression of starch hydrolysis reaction with DE, or dextrose equivalent, is determined by the total amount of dextrose-reducing reducing sugar (D) glucose (calculated as a percentage in the dry matter). Acid hydrolysis is restricted because the color is exceeded if exceeded. Dark and bitter taste appears in the syrup. If acid hydrolysis is performed in dry conditions, the production sticks. In the enzyme-acid method, first a hydrolysis is performed up to a certain DE and then the rest of the process is done with the enzyme. In the multi-enzymatic method, the iodine starch is gelatinized, then the process begins with alpha-amylase and then continues with a mixture of enzymes until it reaches a specific DE. Limited hydrolysis of corn starch (DE) is equivalent to 10 (maltodexirin), which is used as a fat substitute in prepared foods. To produce corn with high fructose, HFCS (first, starch is completely hydrolyzed, then by passing through a column that stabilizes the isomeric glucose embryo, part of the glucose is converted to fructose. To obtain a higher fructose syrup, HFCS syrup They pass through an ion exchange resin that absorbs fructose resin. After separating fructose, a high fructose syrup can be prepared.
3.4.4 - Cellulose
Selobiosis, which is made up of two beta-linked glucose molecules, 1 ----- 4 (is a repeating cellulose unit). Cellulose is resistant to chemicals and natural agents. Cellulose, like amylose, does not have branched starch, but unlike mulch, it does not have a twisted and twisted state, because the hydrogen bonds between glucose and glucose in the cellulose structure are such that it does not allow it to twist and twist. Cellulose chains are placed next to each other, resulting in many hydrogen bonds between these chains. In some parts, the rings are very close together and take on a regular, crystalline shape. While in other parts of the chain they are spaced apart to form a shapeless structure and work. Basically, the absorption of water by cellulose is the same as in the work. Also, these parts react more easily with chemicals such as acid. Due to the hydrolysis of acidic parts of cellulose, microcrystalline crystals are formed from the crystalline parts, which are called oocysts. Ovisil is not absorbed because the body lacks the enzyme cellulose, and is added to food systems to create viscosity. Small particles of cellulose with a diameter of less than a micron are also produced, which give the mouth a fat-like sensation and are produced as fat substitutes under the name of cellulose. One of the cellulose derivatives of carboxymethylcellulose (CMC) is chlorostatic effect on cellulose. It is widely used in food as a thickener, suspension and stabilizer. Methylcellulose is another cellulose derivative that It creates gel at high temperatures, but unlike other gums after Cooling becomes a solution again. [08:59, 01/07/2020] Tanaz: Cellulose, a pistachio coating, is also made from cellulose. In carrots, over time, with the loss of water and the increase in hydrogen bonds between cellulose chains, hard tissue is formed.
3.4.5 - Hemicellulose
Hemicellulose can be present as polysaccharide and heteropolysaccharide. Heme polysaccharides such as glucan and mannan, which are polymers of galactose and mannose, respectively. But most cellulose is made of heteropolysaccharides and is made from sugars such as arabinose, xylose, glucuronic acid and, to some extent, doxycycline. Cellulose in flour increases the water absorption power and improves the mixability properties of flour. They also change the staleness of bread and are considered dietary fiber.
4.6 - Pectin
Pectin polymer is a galacturonic acid that binds to alpha-1 (1 - 4) bonds. They make up the total molecule, but the pectins in nature usually have 12-9% of the methoxyl group. In immature fruits, pectin is called bovine cellulose, called protopectin, and is insoluble in water. If a number of methoxyl groups are separated, pectinic acid is formed, which is soluble, and if all methoxyl groups are separated, pectic acid is formed, which is insoluble. Pectin is used to create viscosity, stability, suspension, and consistency in food systems. Pectin is also used as an emulsifier. Gel formation by pectin: [08:59, 01/07/2020] Tanaz: Pectin can absorb a lot of water due to its high pregnancy rate. Acid and sugar are essential for pectin gel production. By neutralizing carboxyl negatively charged groups, the acid provides the closeness and connection of pectin chains to gel formation. Sugar also provides some proximity to pectin chains by taking some water molecules. It has been said that sugar can also bind between pectin chains. The gel is formed in 3/4 –PH = 2/8. The percentage of sugar required is 65%. Gel above 3.5 does not form a gel. At pH less than 2.8, the gel is formed at high temperatures. Low methylation pectins require less sugar to form gels, provided that there are two-dimensional metals, specifically calcium, in the environment. These bimetallic metals provide the basis for gel formation by joining carboxylic groups together. Pectin with a methylation degree of less than 50%, in the presence of calcium, without the presence of sugar and in the pH range of 2.5 to 6.5 can also form a gel. Pectins with a very low methylation rate and very high) make up less than 50% and more than 70% (rapidly forming gels, respectively), while pectin with a moderate methylation rate (70%) (longer to form a gel) They need. The degree of gel formation is equal to the amount of pounds) or kilograms (sucrose with one pound) or kilograms (pectin in the appropriate acidic conditions of the gel. Usually, the degree of gel formation of pectins in the market is 150-300. Pectin can be extracted from citrus peel and the middle part of the apple. For the production of pectin, it is better than fresh, unripe fruit with fresh ripe fruit. As the pectin fruit increases, the degree of methylation decreases, and the pectin chain may be broken, both of which reduce the strength of the pectin gel.
.4.7 - Gums
Gums are hydrochloric acids that increase the viscosity by absorption and thus increase the stability of some food systems. Gums can also be used as a fat substitute. [09:00, 01/07/2020] Tanaz: Gum arabic: This gum is derived from the acacia plant and, unlike other gums, has low viscosity at low concentrations, but with increasing concentration, the viscosity of the solution increases rapidly. Due to its presence in pregnant women, gum is more stable at high and low pH levels and produces less viscosity. In addition to being stable, these gums also have emulsifying properties. The building blocks are: L-arabinose, L-raffinose, D-galactose and D-glucuronic acid. This gum is placed as a layer on the flavoring substances and by stabilizing them, it prevents them from separating during the processes. This gum is not well compatible with gelatin and tarragon. Targacanth: In Iran it is called tragacanth gum. This gum is composed of D-galacturonic acid, L-focus, D-galactose, D-xylose and L-arabinose units. This gum is very stable in high temperature conditions and acidic environment, so it is used in a variety of sauces such as ketchup. By dissolving this gum in water, a soluble component called tragacanthin and an insoluble part called basiorine are produced. Alginate: Obtained from brown algae. They are derivatives of alginic acid and are composed of beta-D-manic acid and alpha-L-gluloronic acid units. The salts of alkali and ammonia metals of alginates are easily dissolved in hot and cold water, but the salts of metals of two or three capacities dissolve them. It solves. This gum forms a gel in the presence of calcium. Carginan: This gum is a polymer of sulfate hexases and contains 88% galactose and mannose and 5% other carbohydrates. It contains 6% protein and 1% ash. Guar gum: Glucose is obtained from endosperm. Chemically, the gum is made up of beta-D-manopranosil units attached to 1 ---- 4 (connected to each other). These units are connected one by one to a galactopyranosyl unit. Recent connections from Alpha type 1 (1 - 6). This gum forms a concentrated solution with the property of thixotropy. Guar gum is electrically neutral and its viscosity is not affected by pH. Dextran: This gum is microbial and consists of beta units. 1 ---- 6 (Glucan is composed of [09:01, 01/07/2020] Tanaz: Xanthan: This is also a microbial gum that has a very high molecular weight. Its constituents are glucose, mannose and galacturonic acid. It is soluble in hot and cold water and produces very concentrated solutions
Gellan: Produced by Pseudomonas aeruginosa.
References
Food Chemistry, Dr. Hassan Fatemi
Desrosier, N. W. (ed.). 1977. Elements of Food Technology. AVI, Westport, Duckworth, R. B. (ed.). 1975. Water Relations of Foods. Academic Press, London. 12. Eskin, N. A. M. 1990. Biochemistry of Foods, 2nd edn. Academic Press, London. 14. FAO, WHO and UNU Expert Consultation. 1985. Energy and Protein Requirements. Technical report Series 724. World Health Organization, Geneva. 15 - Fellows, P. J. 1988. Food Processing Technology: Principles and Practice. Ellis Horwood, New York. 16 - Fennema, O. R. (cd.). 1976. Principles of Food Science: Part I, Food Chemistry. Marcel Dekker, New York. 17 - Fennema, O. R. (ed.). 1985. Food Chemistry, 2nd edn. Marcel Dekker, New York. 18 - Fennema, O. R. (ed.). 1996. Food Chemistry, 3rd edn. Marcel Dekker, New York. 19 - Fox, P. F., Morrissey, P. A. and Mulvihill, D. M. 1982. Chemical and enzymatic modification of food Proteins. In Developments in Food Proteins, ed. Hudson, B.J. F., Applied Science Publishers, London. 20 - Furia, T. F. (ed.). 1972. Handbook of Food Additives, 2nd edn. CRC Press, Boca Raton, Florida. 21 - Giese, J. H. 1993. Alternative Sweeteners and Bulking Agents. Food Technol. 47(1), 114-126. 22 - Giese, J. H. 1995. Vitamin and Mineral Fortification of Foods. Food Technol. 49(5), 110-122. 23 - Gunstone, F. D. and Norris, F. A. 1983. Lipids in Foods: chemistry, biochemistry and technology. Pergamon Press, Oxford. 24 - Hammond, E. G. 1985. Stability of soybean oil to oxidation. In Proceedings of the World Soybean Research Conference III, ed. R. Shibles, Westview Press, London. 25 - Harris, P. (ed.). 1990. Food Gels. Elsevier Applied Science, London. 26 . Harris, R. S. and Karmas, E. (eds.). 1977. Nutritional Evaluation of Food Processing, 2nd edn. AVI, Westport, Connecticut. 27 - Health, H. B. and Reineccius, G. 1986. Flavor Chemistry and Technology. AVI, Westport, Connecticut. 28 . Heckman, E. 1977. Starch and its modifications for the food industry. In Food Colloids, ed. Graham, H. D., AVI, Westport, Connecticut. 1. Alias, C. and Linden, G. 1991. Food Biochemistry, Ellis Horwood, New York 2. Anon. 1998. INFORM. 9(2), 175. 3 - Aurand, L. W. and Woods, E. A. 1973. Food Chemistry. AVI, Wem Pon, Connecticut 4. Baianu, I. C. (ed.). 1992. Physical Chemistry of Food Procemes, Vol. I, Van Nostrand Reinhold, New York, 5. Belitz, H. D. and Grosch, W. 1987. Food Chemistry, Springer Verlag, Bertio 6 - Bennink, M. R. and Srisuma, N. 1989. Digestibility of dry legume Starch and Protein. In Proceedings of the World Congress on Vegetable Protein Utilization in Human Foods and Animal Foodstuffs, ed. Applewhite, T. P., AOCS Press, Champaign, Illinois. 7. Berk, Z. 1976. Introduction to Biochemistry of Foods. Elsevier Scientific Publishing Company, Amesterdam. 8. Charly, II. 1970. Food Science. The Ronald Press Company New York. 9. Coultate, T. P. 1996. Food-The Chemistry of Its Components, 3rd edn. Royal Society of Chemistry, Cambridge. 10. deMAN, J. M. 1990. Principles of Food Chemistry, 2nd edn. Van Nostram Keinhold, New York
- Service ProvidersBandar Food Industry Knowledge Group
- DateJune 30 2020
- Grouping News and Articles
- Source linkwww.BandrFood..ir
- Subscribe